X chromosome inactivation is a fundamental process in female mammals that elegantly addresses the gene dosage imbalance caused by the presence of two X chromosomes. This intricate mechanism ensures that one of the X chromosomes is silenced, allowing females to have similar levels of gene expression as males, who possess only one X chromosome. Jaunting into this topic reveals a pathway towards understanding genetic disorders like Fragile X Syndrome and Rett Syndrome, conditions that are linked to mutations on the X chromosome. Groundbreaking research led by Jeannie Lee has provided pivotal insights into the chromosomal silencing process, uncovering how Xist RNA transforms the surrounding chromatin environment to inactivate one of the X chromosomes. With the potential to unlock therapies that could alleviate the burdens of these genetic disorders, research into X chromosome inactivation has far-reaching implications for medical science and public health.
The process of X chromosome inactivation, also referred to as dosage compensation in females, plays a crucial role in balancing gene expression levels between the sexes. By inactivating one of the two X chromosomes present in females, this mechanism avoids the overexpression of X-linked genes. Research focusing on the dynamics of chromosomal silencing has gained attention for its implications in various genetic conditions, including Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s investigations into the molecular underpinnings of this process delve into how Xist RNA orchestrates the chromatin restructuring necessary for effective inactivation. As science continues to unravel the complexities behind this genetic switch, the hope for developing innovative treatments for X-linked genetic disorders grows ever stronger.
Understanding X Chromosome Inactivation: The Key to Genetic Disorders
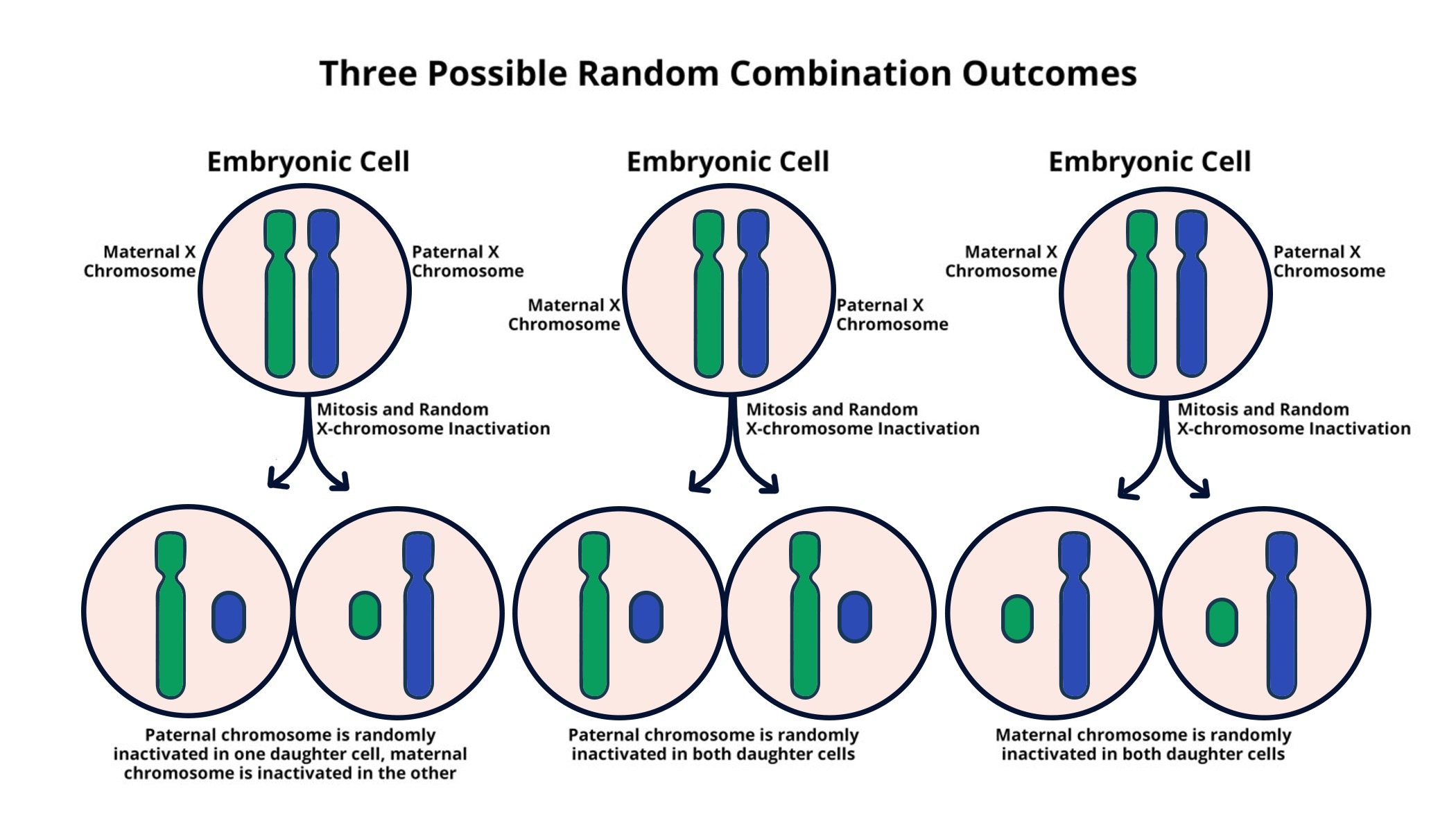
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females in humans. In females, with two copies of the X chromosome, one is randomly inactivated during early development, which balances the gene expression with males who only possess a single X chromosome. This mechanism is vital because it prevents potential overexpression of X-linked genes, which could lead to disorders. Researchers like Jeannie Lee have made significant strides in unraveling the complexities of XCI, highlighting how certain molecular interactions and the unique properties of chromatin contribute to the effective silencing of one X chromosome in females.
One of the remarkable aspects of X chromosome inactivation is its implications for genetic disorders, particularly those linked to mutations on the X chromosome. Conditions such as Fragile X Syndrome and Rett Syndrome emerge predominantly in females due to the malfunction of XCI, where the normal gene on one X can be inactivated while the mutated gene remains active. Understanding the mechanics behind XCI not only sheds light on these disorders but also opens paths for potential therapies that could target and unsilence genes that are otherwise dormant.
The Role of Jeannie Lee’s Research in Chromosomal Silencing
Jeannie Lee’s research at Massachusetts General Hospital marks a pivotal moment in the study of chromosomal silencing, particularly how X chromosome inactivation occurs. Her team’s investigation into the gelatinous substance that surrounds chromosomes—referred to as Jell-O—has revealed how this material alters its properties during XCI. This novel approach highlights the importance of physical interactions within the cell, as the interactions between Xist RNA and Jell-O significantly contribute to the inactivation process, effectively rendering one X chromosome silent. Lee’s insights are critical for advancing our understanding of gene regulation.
The implications of Lee’s work extend beyond basic research; they suggest therapeutic avenues for treating genetic disorders associated with the X chromosome. By potentially reversing the process of inactivation or silencing, researchers could restore function to genes impacted by mutations. This approach anticipates a future where conditions like Fragile X Syndrome and Rett Syndrome may be treatable through innovative techniques derived from the fundamental understanding of chromosomal behavior.
Advances in Gene Therapy for Fragile X and Rett Syndromes: Hope on the Horizon
Emerging therapies for Fragile X Syndrome and Rett Syndrome are particularly exciting as they build on foundational research like that conducted by Jeannie Lee. With insights into X chromosome inactivation, scientists are exploring methods to manipulate and ‘unsilence’ genes that are locked within an inactivated X chromosome. These strategies could offer hope for individuals affected by these conditions, potentially allowing access to functional versions of genes that have been previously rendered inactive. The anticipation surrounding gene therapy hinges not only on the mechanisms uncovered by Lee’s research but also on the evolving landscape of genetic treatment options.
The prospect of translating these research findings into clinical applications holds promise for those impacted by X-linked disorders. Safety studies initiated by Lee’s lab aim to ensure that these treatments can be effectively and safely integrated into existing medical practices. Such developments are pivotal, as they might lead to significant improvements in the quality of life for patients suffering from Fragile X Syndrome and Rett Syndrome. As the field of genetics advances, it becomes increasingly clear that understanding the X chromosome’s behavior is vital for overcoming the challenges posed by genetic disorders.
Future Directions in Research on X-Linked Genetic Disorders
Looking ahead, the future of research surrounding X-linked genetic disorders is bright, particularly with respect to the advancements in understanding X chromosome inactivation. The foundational work by researchers like Jeannie Lee has opened the door to innovative therapeutic approaches that could potentially alleviate the burden of conditions such as Fragile X Syndrome and Rett Syndrome. Continued focus on the molecular mechanisms that underlie XCI will inform future strategies to unsilence genes that currently lie dormant due to their inactivated state.
Future studies are expected to delve deeper into why certain X-linked genes remain unaffected during the unsilencing process. Investigating the capacity of cells to utilize genes is crucial, as it may reveal important information about gene expression regulation not only on the X chromosome but across the genome. As researchers expand their knowledge and develop novel therapeutic methods, the potential to transform the outlook for individuals with X-linked disorders grows exponentially.
The Impact of Chromosomal Silencing on Genetic Research
Chromosomal silencing, particularly that which occurs through X chromosome inactivation, has a profound impact on genetic research and our understanding of gene expression. This phenomenon not only serves to illustrate how cells manage gene dosage but also emphasizes the intricate relationships between molecular biology and genetic disorders. Through continued exploration, researchers are uncovering the therapeutic potential that lies within manipulating chromosomal silencing to reactivate beneficial genes and combat diseases.
For individuals affected by conditions like Fragile X Syndrome and Rett Syndrome, the advancements in understanding chromosomal silencing signify a crucial step toward developing effective therapies. Insights gained from ongoing research can directly inform methods to target and restore gene function, emphasizing the importance of fundamental research in paving the way for practical applications. The field stands at a crossroad where the progression from basic biology to clinical solutions offers hope and revitalization for those affected by genetic disorders.
Exploring the Mechanisms Behind XCI: Key Insights from Jeannie Lee
Exploring the mechanisms behind X chromosome inactivation (XCI) reveals key insights that are crucial not only for basic biology but also for developing treatments for X-linked disorders. Jeannie Lee’s groundbreaking research emphasizes the complex interplay between various molecular components involved in this process. The identification of Xist, an RNA molecule pivotal to XCI, marks a significant milestone in our understanding, as it actively participates in modifying the surroundings of the X chromosome to achieve silencing. This insight into molecular interactions offers a window into the possibilities of gene therapy.
The ability to influence how the Jell-O-like substance around chromosomes behaves during XCI provides potential therapeutic pathways to reactivate genes rendered inactive due to disease. By targeting the molecular dynamics at play and optimizing methods to alter these interactions, researchers can potentially develop interventions that could lead to significant advances in treatment approaches for conditions such as Fragile X and Rett Syndromes. Continued research into these areas highlights the exciting potential for innovative strategies to tackle genetic disorders.
Clinical Implications of X Chromosome Research in Gene Therapy
The clinical implications of ongoing research into X chromosome inactivation (XCI) and its role in genetic disorders are immense. Advancements in our understanding of how genes become silenced on the X chromosome lead to revolutionary therapeutic approaches aimed at reactivating these dormant genes. Jeannie Lee’s research, focusing on the structural and molecular basis of XCI, equips future therapies with the foundational knowledge necessary to address conditions like Fragile X Syndrome and Rett Syndrome effectively. The potential to reactivate beneficial genes carries a transformative capacity for patients suffering from these disorders.
As research progresses, the clinical application of these findings will likely evolve, paving the way for targeted therapies designed to reverse the effects of genetic mutations. The initial steps taken to translate fundamental discoveries into clinical trials offer hope that these novel techniques could drastically improve patient outcomes, reducing or eliminating the symptoms associated with X-linked disorders. Coupled with ongoing safety studies, the path to clinical implementation seems promising, reinforcing the critical nature of continued exploration in the world of genetic therapy.
Collaborative Efforts in Genetic Research: Uniting Science for Progress
Collaboration across various sectors in the field of genetics is fundamental for driving research forward, particularly in complex areas like X chromosome inactivation. The synergy between scientists, clinicians, and research institutions enables a multifaceted approach to studying genetic disorders such as Fragile X and Rett Syndromes. Jeannie Lee’s extensive collaboration with others in genomic research exemplifies how combined expertise can accelerate discoveries and translate findings into actionable therapies, emphasizing the need for interdisciplinary partnerships in genetic research.
Such collaborations not only enhance the breadth of research but also amplify the impact of findings at the clinical level. By uniting expertise from different domains, researchers can share resources, innovative ideas, and techniques that foster significant advancements in understanding diseases and developing treatment options. The cumulative efforts in the scientific community spotlight the paramount importance of working together toward common goals in advancing healthcare solutions for genetic disorders.
The Future of Gene Therapy: Exciting Developments Ahead
The future of gene therapy for conditions linked to the X chromosome is filled with exciting developments, thanks to foundational research conducted by scientists like Jeannie Lee. By understanding the intricacies of X chromosome inactivation and the potential for unsilencing genes, researchers are now positioned to devise groundbreaking treatments for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Ongoing innovations in gene therapy will play an integral role in shaping the therapeutic landscape, making previously untreatable conditions manageable.
As research continues to unravel the complexities involved in chromosomal silencing, the therapeutic applications of these insights are expected to expand. Developing targeted treatments that utilize the body’s own mechanisms to re-engage silenced genes represents a paradigm shift in how genetic disorders may be approached in the future. The exciting horizon of gene therapy holds the promise of revolutionizing not just treatment paradigms for X-linked disorders, but the entire field of genetics, offering hope and healing for many.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation (XCI) is a crucial biological process where one of the two X chromosomes in female cells is inactivated to ensure dosage compensation between males (who have one X chromosome) and females. This process is vital because it prevents males and females from producing different amounts of proteins encoded by genes on the X chromosome. Deciphering how XCI works provides insight into X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome, offering potential avenues for treatments.
How does Jeannie Lee’s research advance understanding of X chromosome inactivation?
Jeannie Lee’s research at Mass General has made significant strides in understanding the mechanisms behind X chromosome inactivation. Her studies revealed how the Xist RNA molecule interacts with a gelatinous substance surrounding chromosomes, effectively leading to chromosomal silencing. This work is fundamental in exploring therapeutic options for genetic disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
What role do mutations on the X chromosome play in diseases like Fragile X Syndrome?
Mutations on the X chromosome, such as those causing Fragile X Syndrome, can lead to various intellectual and developmental disabilities. X chromosome inactivation means that in females, one of the X chromosomes carries the mutation while the other may have a healthy version of the gene. Understanding X inactivation is key to developing therapies aimed at reactivating these silent healthy genes, potentially alleviating the effects of mutations.
Can X chromosome inactivation methods be applied to treat Rett Syndrome and Fragile X Syndrome?
Yes, methods derived from research on X chromosome inactivation—particularly those that focus on unsilencing inactivated X-linked genes—are being explored as potential treatments for Rett Syndrome and Fragile X Syndrome. Jeannie Lee’s lab is optimizing these approaches, aiming to move towards clinical trials that could significantly improve the lives of individuals suffering from these genetic disorders.
What is the significance of chromosomal silencing in the context of XCI?
Chromosomal silencing is a critical aspect of X chromosome inactivation, where one X chromosome in female cells is rendered inactive to balance gene dosage between sexes. This silencing mechanism helps control gene expression and can prevent certain genetic disorders that arise from X-linked mutations. Jeannie Lee’s research highlights how the interaction between Xist RNA and surrounding chromosomal ‘Jell-O’ leads to effective silencing, paving the way for future therapeutic strategies.
How can understanding X chromosome inactivation lead to breakthroughs in genetic disorder treatments?
By understanding the precise mechanisms of X chromosome inactivation, researchers aim to free inactivated X chromosomes harboring healthy genes. This ‘unsilencing’ could allow cells to utilize these genes, offering potential cures for diseases like Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s findings, particularly on how Xist interacts with chromosomal environments, are central to developing effective therapeutic interventions.
| Key Point | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes while males have one, necessitating the inactivation of one X in females to avoid excess gene dosage. |
| Role of Xist | The Xist gene produces an RNA molecule that initiates the inactivation process by altering the material properties of the surrounding chromosomal coating. |
| Jell-O-like Substance | A gelatinous material coats chromosomes, which facilitates cellular organization and the process of X-inactivation. |
| Therapeutic Potential | Research may allow for the unblocking of mutated genes in disorders like Fragile X Syndrome and Rett Syndrome, leading to potential treatments. |
| Future Directions | Clinical trials are planned to optimize therapies targeting X-linked genetic disorders based on these findings. |
Summary
X chromosome inactivation is a vital process in female mammals that ensures gene dosage balance between sexes. This complex mechanism, where one of the two X chromosomes in females is rendered inactive, has been the subject of extensive research. Recent breakthroughs elucidating this process offer promising avenues for treating genetic disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Understanding how X chromosome inactivation occurs opens up therapeutic possibilities of unsilencing mutated genes, marking a significant step forward in genetic research and treatment.